Open Journal of Biological Sciences
The Neuroprotective Effect of Calotropis procera Against Toxicity of Mercury Chloride
Belfarhi Leila1* and Bairi Abdelmadjid2
1Permanent Researcher at the CRAPC Research Center in Algiers, Algeria
2Processor and Director of Laboratory of Applied Neuro-Endocrinology, Department of Biology, Faculty of Science, University of Badji, Moukhtar-Annaba-B.P.12, Annaba-23000, Algeria
Cite this as
Leila B, Abdelmadjid B. The Neuroprotective Effect of Calotropis procera Against Toxicity of Mercury Chloride. Open J Biol Sci. 2024;9(1):004-007. Available from: 10.17352/ojbs.000038Copyright
© 2024 Leila B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Background: Central nervous system intoxication can result from exposure to various toxins, including mercury chloride. Although several chelating agents are available for mercury chloride detoxification, their efficacy can diminish over time. Calotropis procera, a medicinal plant, has shown potential as a protective agent against mercury chloride-induced brain damage. This study aims to evaluate the protective effects of Calotropis procera in mitigating mercury chloride toxicity.
Experimental animal design: This study investigates the protective effects of Calotropis procera against mercury chloride toxicity in Wistar albino rats. A total of 36 rats, comprising both males and females, were housed under controlled laboratory conditions and divided into two main groups based on five animals. Each group was further subdivided into five subgroups: a control group, a group treated with Calotropis procera, a group treated with mercury chloride, a group treated with both mercury chloride and Calotropis procera and a group receiving Calotropis procera alongside mercury chloride. Treatments were administered for 20 days. After the treatment period, the rats were euthanised, and brain tissues were collected for histopathological analysis. After the brain tissues were fixed in 10% saline-buffered formalin, they were processed through a series of ascending grades of ethanol to dehydrate them. The tissues were then cleared in xylene and embedded in paraffin. The paraffin-embedded brains were treated three times with pure paraffin to ensure proper infiltration and were subsequently moulded into blocks. Sections of 5 µm thickness were prepared using a Leica microtome and stained with Haematoxylin and Eosin (H&E) for histopathological examination. The study adhered to ethical guidelines and was approved by the relevant regulatory body. The results of this study demonstrated that mercury chloride caused significant cerebral toxicity, manifesting as inflammation and pyknosis of the nuclei. Calotropis procera reduced mercury toxicity and preserved the nuclei in male rats. In female rats, Calotropis procera completely preserved the brain tissue.
Introduction
The use of mercury has a long history, including its notorious rôle in poisoning Agnès Sorel, the mistress of Louis XIV [1]. Historical analyses have also indicated that Isaac Newton suffered from mercury exposure, as evidenced by bone examinations [2]. A major mercury poisoning crisis occurred in Minamata, Japan, where mercury discharge from a chemical factory led to severe health consequences. This tragedy marked the onset of new neurological diseases, the mechanisms of which remained poorly understood for a long time. However, recent studies have provided new insights into the mechanisms of mercury-induced damage to the brain, with a particular focus on Minamata Bay. Advanced analytical techniques, such as high-resolution synchrotron X-ray absorption spectroscopy [3], have revealed that mercury preferentially binds to sulphur groups. This binding action leads to cerebral lesions by disrupting cellular functions, as mercury’s interaction with sulphur interferes with normal biological processes. The Minamata tragedy not only exposed the severe neurological diseases caused by mercury poisoning but also marked the beginning of our understanding of the neuropathological mechanisms associated with mercury exposure. This awareness has been crucial in understanding similar mechanisms in other contexts. Investigating the properties of medicinal plants in relation to mercury toxicity could open new avenues for treating these neurological conditions. Detoxifying mercury from the brain can eliminate this toxic substance, but the resulting damage is challenging to repair with chemical chelators. However, there are natural ways that can help in repairing neurotoxic lesions caused by mercury chloride. To detoxify the mercury chloride, more toxic plants similar to it can be used but in a bénéficial way. Among these plants, there is Calotropis procera which is able to import new voices of detoxification of the mercury chloride. Calotropis procera. It is a plant known in the Algerian Tuareg population as “Torha”. Calotropis procera, commonly referred to as “Torha” among the Algerian Tuareg population, is a perennial shrub characterized by its opposite, large, ovate leaves. The flowers are violet in color. This plant typically grows in Ilizi and has a compact, shrubby form. This plant is characterized by the presence of a white liquid that circulates in all parts of the plant. This liquid is the latex of Calotropis procera. It contains cysteine proteins, rich in thiol groups, which have been widely implicated in the detoxification of heavy metals. It is a plant characterized by a strong odor, giant green leaves, and filled inside with white milk. Calotropis procera is characterized by the presence of several antioxidant components. It contains sweet components like Calotropin and Calotoxin both helping protect neuronal cells from inflammatory damage. This anti-inflammatory action is complemented by rutin and quercetin, which provide strong antioxidant benefits. Oleanolic Acid contributes by stabilizing cell membranes, which minimizes oxidative stress and inhibits neuroinflammation, enhancing the protective effects of the other compound [4]. The plants Calotropis procera contain also Alkaloids that may exhibit analgesic, antidiabetic, and anti-inflammatory properties. Glycosides: characteristics of these compounds can have various biological activities and potential benefits like antioxidants and cardioprotective effects. Saponins: its characteristics help lower cholesterol levels and boost immune function. It also contains flavonoids with anti-cancer and anti-inflammatory activities. Tanin polyphenolic compounds that can bind to proteins. Potential benefits include antimicrobial effects and wound healing. The objective of this study is to explore new natural methods to reduce mercury chloride toxicity in the brain, potentially offering new therapeutic strategies for neurological conditions associated with mercury exposure.
Materials and methods
Plant material
Leaves and roots of Calotropis procera were collected in March 2017 from the Sahara region of Ilizi, Algeria. The plant was initially identified by the local Tuareg community in Ilizi and later verified by a phytochemistry and botany expert from the Animal Department at Badji Mokhtar University, Annaba. The collected plant material was shade-dried in coffee linen bags in a spacious, dark room.
Preparation of extract
A total of 500mg of air-dried and powdered aerial parts of Calotropis procera (Apocynaceae) were extracted with ethanol (80:20 v/v) for 24 hours, repeated three times [5]. After filtration, the filtrates were combined and concentrated at room temperature, then suspended in 900 ml of water. The resulting solution was dried using Na2SO4, filtered, and further concentrated at room temperature to yield 57 g of dry powder from Calotropis procera.
Experimental animal design
Male and female albino rats Wistar weighing 250g obtained from Pasteur Institute (Algiers) were reared in the animal house of the University of Badji Mokhtar-Annaba. They were kept in the laboratory under constant conditions of temperature (24 ± 2 °C) for one month before and through the experimental work, being maintained on a standard diet and water was ready ad-libitum.
The experiment utilized 30 rats, organized into two primary groups of 15 males and 15 females each. These groups were further subdivided into five subgroups, with each subgroup containing three rats. This design follows two of the three Rs of ethical animal experimentation—reduce and refine—as proposed by Russell and Burch [6], focusing on minimizing the number of animals used while improving the conditions of the experiment.
The first group Healthy control group received distilled water for 20 days, while the second group the animals received (200mg /Kg) of Calotropis procera by gavage. The third group received mercury chloride at a dose of 0,2mg/ Kg by gavage, the fourth group received (200 mg /kg) of Calotropis procera and (0,2 mg/ kg) by gavage, and the fifth group were respectively plants Calotropis procera and mercury chloride treated with 1 g/kg/day (Eth1) and 2 g/kg/day (Eth2) of ethanol. The rats of the fifth (Eth1+SMI) and the sixth (Eth2+SMI) groups were firstly treated respectively with 1g/kg/day and 2g/kg/day of ethanol, after one hour, animals were given SMI (200 mg/kg/day). After fixation of brain tissues in 10% saline buffered formalin, the brain tissues were dried in ascending grades of ethanol, cleared in xylol, and then immersed in paraffin. The impregnated brain was treated three times in pure paraffin to be established in blocks. Sections (5 µm thick) were preparatory using Leica microtome and stained by Hematoxylin and Eosin (H&E) for histopathological investigation [7].
Results and discussion
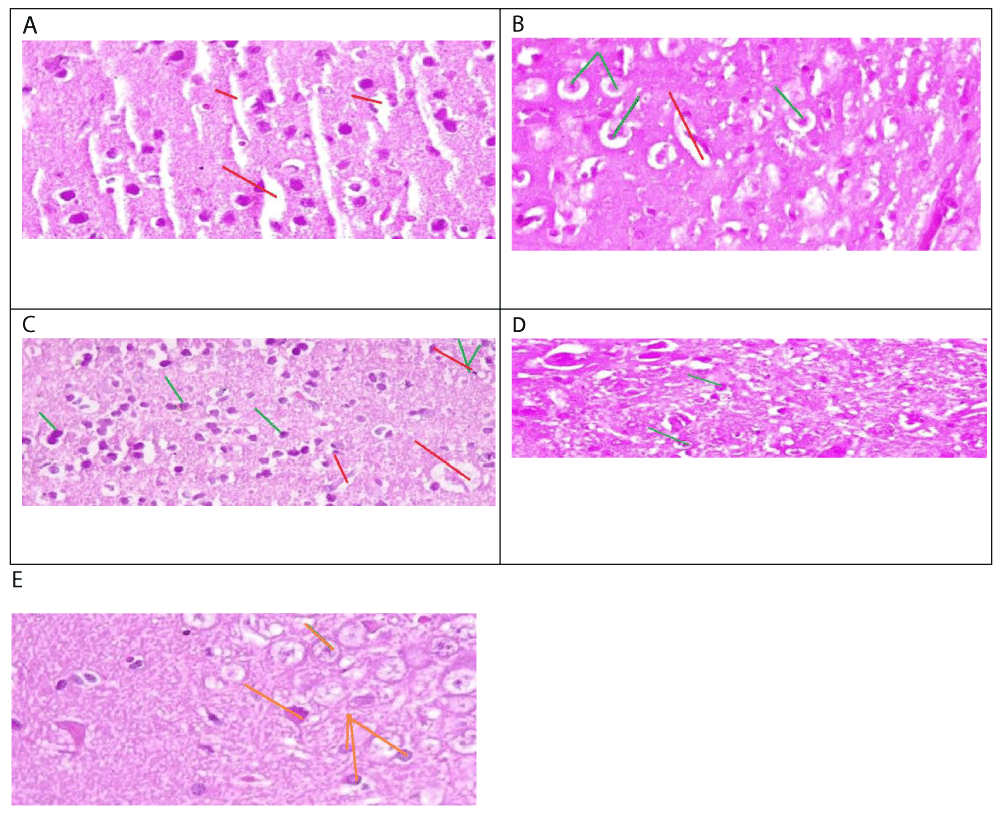
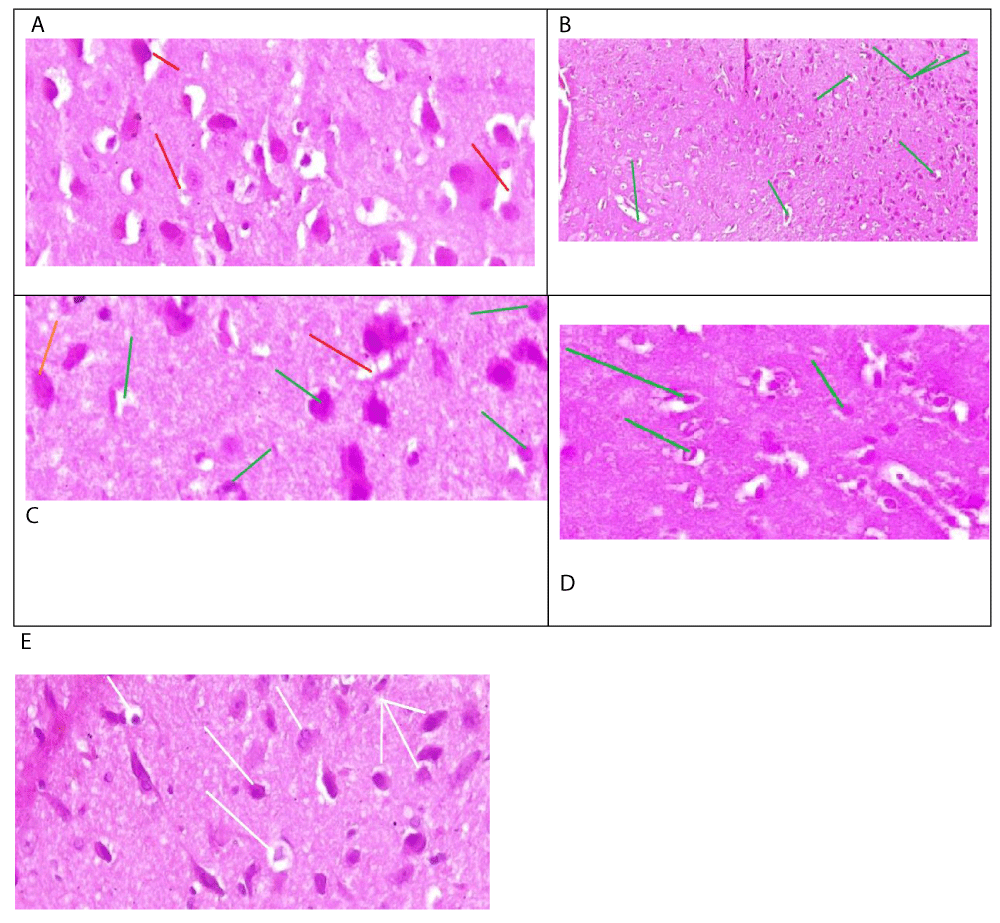
The histological analysis of this study revealed the protective role of Calotropis procera in mitigating the toxic effects of mercury chloride on the brain. Mercury chloride is a potent neurotoxin that induces severe cerebral toxicity, manifested by significant cytoplasmic and nuclear alterations, such as the observed densified cellular nuclei with laminated chromatin in male rats (Figure 1a). Histological results from male rats treated with both mercury chloride and Calotropis procera demonstrated complete preservation of brain tissue against mercury-induced toxicity (Figure 1b). Examination of brain tissue sections from male rats treated with Calotropis procera and mercury chloride revealed occasional images of karyolysis associated with edema, particularly within the plexiform or molecular layers. However, the cytoplasmic contours were preserved, and cellular nuclei remained intact (Figure 1C). The histological studies of male rats treated by Calotropis procera demonstrated subnormal morphology of brain tissues (Figure 1D). Histological analysis of brain tissue from female rats treated with mercury chloride showed scattered nuclear pyknosis and increased nuclear density, along with laminated chromatin (Figure 2a). Conversely, tissue sections from female rats treated with both Calotropis procera and mercury chloride exhibited well-preserved nuclei (Figure 2C). Microscopic examination of the brain tissue from female rats treated with mercury chloride and Calotropis procera also revealed signs of pyknosis and nuclear densification, with well-preserved nuclei in other regions of the brain tissue (Figure 2C). Brain tissue from female rats treated with Calotropis procera exhibited subnormal cerebral morphology (Figure 2D).
Our study, which involved both male and female rats, investigated the protective effect of Calotropis procera against the neurotoxic effects of mercury chloride. We found that mercury chloride-induced severe cerebral toxicity in both sexes, characterized by significant cytoplasmic and nuclear alterations. Previous research has shown that mercury chloride causes necrosis of nerve cells affecting the cerebral cortex, hypothalamus, and cerebellum [8]. The effects of mercuric chloride can be irreversible at certain lethal doses. In cases of acute poisoning, a patient who ingested a dose of 7 g of mercuric chloride died within six days, despite treatment with chelators [7]. This illustrates that high doses of mercury can have fatal effects in a limited time frame. A second case report describes two instances of mercury intoxication involving different doses. The first case involved a 34-year-old man who received 40 g of mercury intravenously over 11 days. He was treated in the hospital with gastric lavage and chelator therapy. In contrast, the second case resulted in death due to severe dyspnea and encephalopathy. These cases highlight the neurotoxic effects of mercury, which can be lethal despite the treatments administered in the first patient’s case [9]. Studies indicate that long-term exposure to levels of 0.01 mg/m³ over several months can impair memory and concentration, and lead to anxiety disorders [10]. Effects have been observed in individuals exposed to low levels for durations of 6 to 12 months. Another study reports the emergence of symptoms indicative of mercury-induced brain toxicity, such as headache, dizziness, convulsions, and fatigue [11]. The dose responsible for this effect is 1mg. Other studies have also reported that mercury chloride induces necrosis and apoptosis of neurons and astrocytes in the motor cortex [7]. These studies indicate that even low exposure to mercury chloride can impair nerve cells. Our results also demonstrated that within the plexiform layer of brain tissue from male rats treated with both Calotropis procera and mercury chloride, there were instances of karyolysis associated with edema, while cytoplasmic contours and cellular nuclei were conserved. Similar effects were observed during the Minamata incident in Japan, where mercury chloride was found to preferentially affect the plexiform molecular layer of the brain [12]. Autopsy studies of mercury-intoxicated animals revealed karyolysis of nuclei, as well as vascular damage in subcortical brain regions, including edema, vessel damage, widening of perivascular spaces, and infiltration of pale red fluid [13]. Other studies have also identified karyolysis leading to neuronal damage and a decrease in neuron count [14].
Our findings indicate that Calotropis procera reduced the toxic inflammatory effects caused by mercury chloride and protected cellular integrity by preserving cytoplasmic contours and cellular nuclei within brain tissue. Phytochemical investigations of this Saharan plant have highlighted its richness in flavonoids, such as rutin [15]. Rutin has been shown to reduce brain lesions observed in cancer [16,17], which may explain its neuroprotective role against mercury chloride-induced toxicity observed in our study. Additionally, stilbene, another compound in Calotropis procera, can halt the cascade of cerebral inflammatory reactions. Stilbene also protects against brain lesions and inflammation by stimulating the expression of the enzyme heme oxygenase. Histological results from male rats treated with both mercury chloride and Calotropis procera demonstrated that the plant completely protected brain tissue from mercury chloride toxicity. This protective effect of Calotropis procera is attributed to its wealth of molecules that target toxic brain proteins. For example, oleandrin, a neuroprotective molecule, inhibits the expression of the toxic alpha-synuclein protein, thereby preventing brain lesions associated with this protein’s toxicity.
Conclusion
This study investigates a plant called Calotropis procera and its effect on reducing the effect of mercury chloride. The result demonstrates that Calotropis procera can reduce the inflammatory reaction in both male and female rats. Calotropis procera has been shown to preserve cell integrity by protecting the cellular nuclei despite the toxic effects of mercury chloride. This effect is due to the antioxidants in Calotropis procera, which were synthesised under high heat conditions in the Sahara Desert of Algeria. This harsh climate has led to the formation of molecules that have been able to penetrate the brain and counteract the effects of mercury chloride. Although the toxicity of mercury chloride is well-known, this study suggests that Calotropis procera could open new avenues for the treatment of inflammatory brain diseases.
This study on the neuroprotective effects of plants against mercury toxicity was conducted with a small sample size of animals, and histological analyses were performed using optical microscopy. While these techniques demonstrated observable effects, the intrinsic and molecular mechanisms of the plant Calotropis procera require more advanced methods, such as scanning electron microscopy (MEB). Investigate the effects of Calotropis procera extracts on brain cell cultures to elucidate the cellular pathways involved in neuroprotection.
Ethical considerations
The study adhered to ethical guidelines and was approved by the relevant regulatory body of the University of Badji Mokhtar-Annaba.
- Ray MC. Mercury, a toxic element. Future. Available from: https://www.futura-sciences.com/sante/dossiers/biologie-poisons-histoire-%201676/page/10/
- Johnson LW, Wolbarsht ML. Mercury poisoning: a probable cause of Isaac Newton's physical and mental ills. Notes Rec R Soc Lond. 1979;34(1):1-9. Available from: https://www.jstor.org/stable/531510
- James AK, Nehzati S, Dolgova NV, Sokaras D, Kroll T, Eto K, et al. Rethinking the Minamata tragedy: what mercury species was really responsible? Environ Sci Technol. 2020;54(5):2726-2733. Available from: https://doi.org/10.1021/acs.est.9b06253
- Bodakhe N, Kumar A. Neuroprotective potential of Calotropis gigantea: a review. J Pharm Pharmacol. 2016;68(10):1270-1280.
- Ozenda P. Flora of the northern and central Sahara. Paris: National Center for Scientific Research; 1958;400. Available from: https://www.scirp.org/reference/referencespapers?referenceid=453614
- Russell WMS, Burch RL. The principles of humane experimental technique. London: Methuen; 1959;238. Available from: https://www.cabidigitallibrary.org/doi/full/10.5555/19592204037
- Dardouri K, Haouem S, Gharbi I, Sriha B, Haouas Z, El Hani A, et al. Combined effects of Cd and Hg on liver and kidney histology and function in Wistar rats. J Agric Chem Environ. 2016;5(5):159-169. Available from: http://dx.doi.org/10.4236/jacen.2016.54017
- Zaher AM, Taha MMM, Ahmed HG. Neurodegenerative disorders associated with mercuric chloride toxicity in mice and the role of some antioxidants. Int J Sci Res (IJSR). 2015;6(4):391. Available from: https://www.ijsr.net/archive/v6i4/ART20172479.pdf
- Vardhan V, Garg S. Mercury toxicity: a case report. Med J Armed Forces India. 2005;61(1):76-78. Available from: https://doi.org/10.1016/s0377-1237(05)80127-3
- Lu Q, Liu Z, Chen X. Mercury poisoning through intravenous administration: two case reports with literature review. Medicine (Baltimore). 2017;96(46):e8643. Available from: https://doi.org/10.1097/md.0000000000008643
- Kim J. Long-term exposure to mercury and cognitive decline: evidence from a cohort study. Int J Environ Res Public Health. 2022;19(7):4312.
- Zhang X. Cumulative neurotoxic effects of mercury: a review of recent literature. Environ Toxicol. 2023;38(1):1-10.
- Møller-Madsen B. Localization of mercury in CNS of the rat V: inhalation exposure to metallic mercury. Arch Toxicol. 1992;66:79-89. Available from: https://link.springer.com/article/10.1007/BF02342499
- Trakhtenberg D. Chronic effects of mercury on organisms. DHEW Publication. 1974.
- Suzuki T, Imura N, Clarkson TW. Advances in mercury toxicology. New York: Springer Science & Business Media; 1991. Available from: https://books.google.co.in/books/about/Advances_in_Mercury_Toxicology.html?id=pMVezB0Uo0MC&redir_esc=y
- Oraibi AI, Hamad MN. Phytochemical investigation of flavonoids of Calotropis procera in Iraq: isolation and identification of rutin, quercetin, and kaempferol. J Pharm Sci Res. 2018;10(9):2407-2411. Available from: https://www.jpsr.pharmainfo.in/Documents/Volumes/vol10Issue09/jpsr10091861.pdf
- Pathak S. Ruta 6 selectively induces cell death in brain cancer cells but proliferation in normal peripheral blood lymphocytes: a novel treatment for human brain cancer. Int J Oncol. 2003;23(4):975-982. Available from: https://pubmed.ncbi.nlm.nih.gov/12963976/
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
