Annals of Marine Science
Microbial communities around seeds promote Zostera marina germination
Yuki Nakashima1,2, Takumi Sonobe2 and Masataka Kusube3*
2Advanced Engineering Faculty, National Institute of Technology, Wakayama College, Gobo, Wakayama, Japan
3Department of Applied Chemistry and Biochemistry, National Institute of Technology Wakayama College, Gobo, Wakayama, Japan
Cite this as
Nakashima Y, Sonobe T, Kusube M (2024) Microbial communities around seeds promote Zostera marina germination. Ann Mar Sci 8(1): 014-018. DOI: https://dx.doi.org/10.17352/ams.000045Copyright License
© 2024 Nakashima Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Eelgrass meadows are vital not only for sustaining marine biodiversity as marine ecosystems but also for carbon fixation as blue carbon. However, the national decline in eelgrass meadows in Japan, which was initiated by economic growth in the 1980s, prompted the implementation of conservation measures. This study explored the fundamental techniques of the sediment environment to enhance Zostera marina seed germination rates. We investigated the microbial origin of Zostera marina seeds under anaerobic conditions and assessed their germination efficiency. Anaerobic sediments in the eelgrass meadows were found to increase eelgrass germination rate by 6% more than aerobic sediments. And interestingly, the sulfate-reducing bacteria Desulfovibrionaceae and Sulfurospirillaceae were detected mainly on eelgrass seeds. Our result clearly indicated that the sulfate-reducing bacteria around Zostera marina seeds create anaerobic conditions that promote germination through their sulfate-reducing function. Therefore, when developing new eelgrass meadows, it can be expected to improve germination rates by preparing the sediment environment to facilitate the increase of SRB coexisting with seeds. However, the difference in bottom sediment conditions varies by region, so it is necessary to be careful when adding or removing something. In addition, germination and growth of eelgrass must be considered separately, and factors that promote growth are not described in this work. The result of this study was an important finding for the implementation of Blue Carbon activities to improve sediment quality prior to the creation of eelgrass meadows and can also support eelgrass meadows maintenance after establishment.
Introduction
Zostera marina also known as eelgrass is the most widely distributed marine flowering plant in the northern hemisphere [1]. They are important ecosystems in coastal environments that provide geological stabilization through stolon growth and enable marine organisms to spawn in eelgrass meadows [2,3]. These days, marine environments are attracting worldwide attention as a source of blue carbon, natural positive capital, and blue natural capital [2]. In recent years, there has been growing interest in the Zostera marina, with active restoration efforts underway worldwide to enhance eelgrass meadow preservation. For example, to facilitate large-scale restoration of eelgrass meadows, a technique was developed for transplanting mature or adult seagrass plants [4-6]. Transplanting seedlings to restore eelgrass meadows can maintain the genetic diversity of the Zostera marina. However, this method is highly labor-intensive and costly and the establishment rate of seedlings is very low due to the influence of numerous environmental factors. On the other hand, broadcast seeding in the field results in a very low germination rate compared to transplanting adult plants. This is due to some seeds failing to germinate and others being lost to predation by animals, leading to significant seed loss [7-10]. In addition to this, lab-scale studies focusing on Zostera marina seeds have demonstrated that various approaches can enhance the germination rate of these seeds [11,12]. For instance, Morita et al. highlighted the presence of density gradients within Zostera marina seeds, revealing that they land in a specific orientation during free fall, promoting favorable germination positions. Another approach involves the vernalization of seeds, resulting in an enhanced germination rate, emphasizing the intrinsic germination potential of the seeds [13]. However, to effectively preserve eelgrass meadows with a high establishment rate in real marine environments, it is necessary to investigate the preferred germination conditions of the natural Zostera marina. To date, we have conducted detailed analyses of the chemical composition and microbial communities in natural eelgrass meadows along the Japanese coast. Our findings revealed that eelgrass meadows construct a more reductive environment in their sediment compared to bare areas and host numerous sulfate-reducing bacteria [14]. However, how these sulfate-reducing bacteria gather around Zostera marina seeds and establish an anaerobic environment remains unknown. Therefore, this study aims to identify the bacterial origins responsible for constructing the anaerobic environment necessary for the germination of Zostera marina.
Materials and methods
Sampling and seed selection
Zostera marina seeds and anaerobic and aerobic sediments were collected from the Katakui coast of Wakayama Prefecture (33.939789N, 135.084983E). Oxidation-Reduction Potential (ORP) and dissolved oxygen (DO) were measured to directly confirm the conditions in each sediment. ORP was measured using a portable water quality meter (LAQUA D-210C and D-210PC, HORIBA Co.), and DO was measured using a digital DO meter PDO-520 (FUSO Co.). Zostera marina seeds were collected from natural eelgrass meadows on the Katakui coast and performed post-ripening treatment in a natural sea environment for approximately 2 months. High-density seeds were selected by washing saturated saline solution and stored in artificial seawater at 5 °C in a dark place until use.
Effect of anaerobic sediment on Zostera marina germination
Five of each of the above-selected seeds were planted in 40 g of natural anaerobic (ORP: -220 mV, DO: 0.85 mg/L) and aerobic sediment (ORP: +153 mV, DO: 16.0 mg/L) (n = 40 each). These sediments were collected from natural eelgrass meadows from which seeds were collected. Germination rates were monitored in a 60 cm seawater tank set at 10 °C for about 3 months when fluctuations in germination rates ceased. Based on the research results of Xu, et al. we set the germination temperature taking into account the water temperature at the collected locations during the germination period in winter. Common artificial seawater was continuously filtered and circulated through activated carbon and porous ceramic filters [15,16]. LED white light was used for illumination, which was switched every 12 hours between day and night by timer control. In this experiment, germination was defined as the timing of the change from initial white shoots to green shoots, and all germination numbers were counted.
In vitro investigation of bacterial flora formation around seeds
Observations were made under the conditions shown in Table 1 to confirm the formation of the germination environment around the seeds. To identify the sources of the bacterial flora that contribute to germination, multiple patterns of autoclave sterilization of natural sea sand, natural seawater, and seeds were performed. Three seeds were placed separately in plant culture test tubes in each condition (n = 5) and observed in a 60 cm seawater tank each. The temperature during the treatment was set at 10 °C, no LED lighting was installed, and seawater was treated for one month with activated carbon and porous ceramic filter circulation.
DNA extraction and sequencing
The total genomic DNA was extracted from the collected sediment and Zostera marina seeds using an ISOIL for Beads Beating kit (NIPPON GENE Co.) according to the manufacturer’s instructions. The V4 hypervariable region of the 16S rRNA genes was amplified using the 515F (5’-GTGCCAGCMGCCGCGGTAA-3’) and 806R (5’-GGACTACHVGGGTWTCTAAT-3’) primers. DNA polymerase was used for amplification under the following conditions: 95 0C for 5 min; 30 cycles of 95 0C for 20 s, 65 0C for 20 s, 72 0C for 5 s; and 72 0C for 10 min [17,18]. Sequencing libraries were constructed using the MiSeq Reagent Kit V2 (500 cycles) (Illumina Co.) according to the manufacturer’s instruction, and assessed library quality on a Qubit 2.0 fluorometer and 2% agarose gel electrophoresis. Finally, the sequencing library was sequenced on the Illumina MiSeq platform and generated paired-end sequence reads.
Bacterial flora analysis in in vitro investigation tubes
After quality passed and trimmed sequence reads were processed using the Qiime2 software (ver.2021.8). The DADA2 algorithm was used to denoise and merge to generate tables of amplicon sequence variants. The sequences were clustered into operational taxonomic units with 99% sequence similarity using VSEARCH and chimeras were removed by using USEARCH software (ver. 6.1.544) [19]. The taxonomic operational taxonomic units were linked by mapping the 16S rRNA gene amplicons to the Silva database (ver.138) which was used as the reference database. Community dissimilarities were estimated by calculating the Bray–Curtis distance, which is based on the relative abundance of each taxon at different levels [20].
Results and discussion
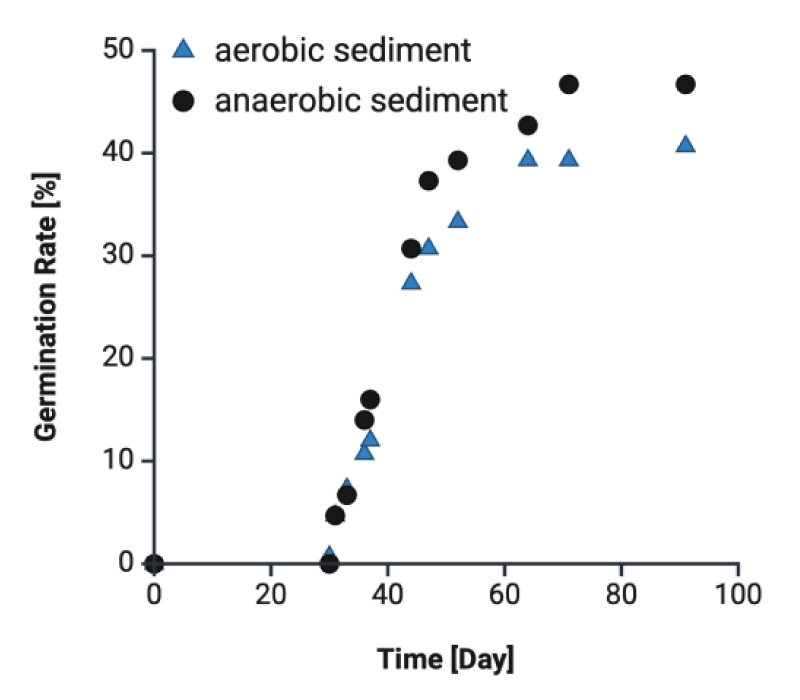
Our previous study has shown that the rhizosphere in eelgrass meadows is constructed in a highly anaerobic environment compared to bare sediment [21]. In addition to this, it has been reported that the anaerobic environment enhanced the germination rate compared to aerobic conditions. Therefore, in this study, we constructed an anaerobic environment around the seeds under different conditions in order to clarify the origin of the microorganisms and how to construct the anaerobic environment. Figure 1 shows similar initial germination rates for several sediments over a month. The x-axis represents the number of days after seeding and the y-axis indicates the average germination rate (200 seeds). In our laboratory-scaled test, final germination rates by 6% under anaerobic sediments than those in aerobic sediments after 45 days, and no difference was observed in the germination timing between both conditions. It is controversial, which primary factors promote seed germination of Zostera marina. Reports have shown that low salinity (5 - 10 psu), low water temperature (< 15 °C), stratification, anoxia, and scarification of the seed coat have also been reported as having positive effects on seed germination [22]. We suggest that anaerobic conditions are favorable for the germination of Zostera marina, particularly in the surrounding environment. And origin of the bacterial flora and its relationship to anaerobic conditions was revealed. However, Jørgensen, et al. 2019 described that the mechanisms behind the construction of anaerobic environments conducive to germination remain unknown [20].
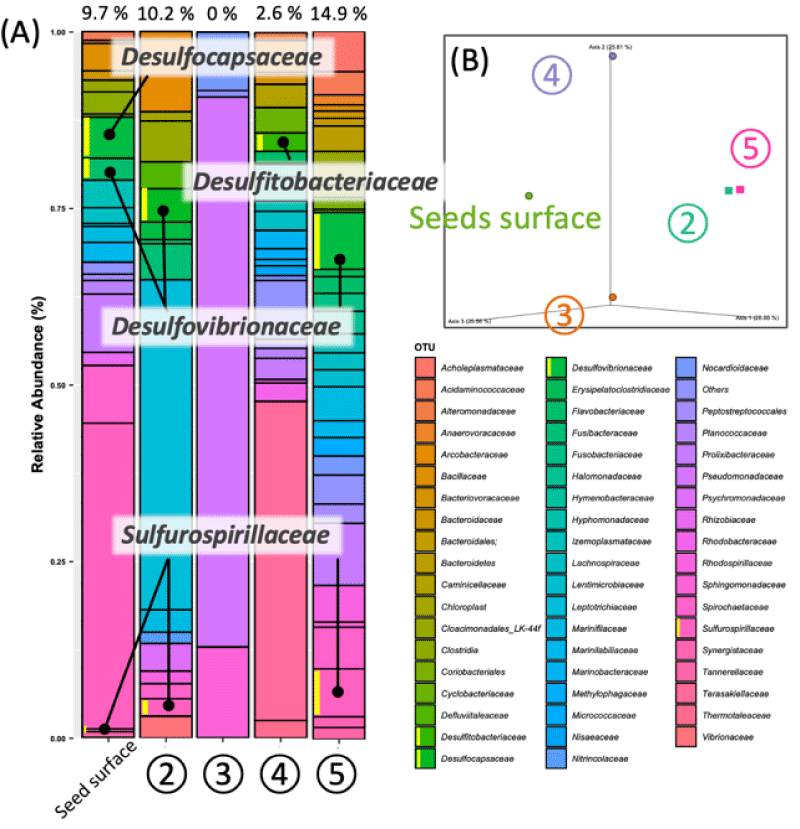
To elucidate the mechanisms of anaerobic environment construction, we investigated the anaerobic conditions surrounding Zostera marina seeds under various conditions. It has been widely reported that diverse anaerobic bacteria inhabit marine environments and that anaerobic conditions are primarily constructed through sulfate reduction utilizing SO42- [23]. Therefore, we aimed to identify the origins of these bacteria to prepare anaerobic conditions that influence the germination of Zostera marina. Based on previous studies, the reductive sediments of seagrass beds are characterized by their black coloration and sulfurous odor. These changes were used as indicators to evaluate the presence of anaerobic conditions. Non-sterile Zostera marina seeds showed black discoloration around the seeds (Figure 2, tubes #2 and #5). No changes were observed in any other sterilization conditions, including the negative control. These results revealed that bacteria originating from Zostera marina seeds are involved in the construction of anaerobic environments.
Bacterial community analysis of seawater, marine sand, and Zostera marina seeds was conducted to determine the mechanism of the formation of anaerobic conditions around the seeds. Figure 3(A) shows the relative abundance of the bacterial communities under each preparation condition. Sulfate-Reducing Bacteria (SRB), which create anaerobic marine environments, such as Desulfitobacteriaceae, Desulfocapsaceae, Desulfovibrionaceae, and Sulfurospirillaceae have been detected [24,25]. These SRBs constituted 9.7% of the total around Zostera marina seed (Figure 2, tube #2) and 10.2% under conditions where components other than the seeds were sterilized (Figure 2, tube #5). Moreover, typical SRB of Desulfovibrionaceae and Sulfurospirillaceae were detected in fresh Zostera marina seeds. This means that sulfate-reducing bacteria present in eelgrass seeds play an important role in constructing the germination environment for Zostera marina seeds. Figure 3(B) shows a β-diversity based on the Bray–Curtis distance considering the relative abundance of each bacterial composition. This illustrates the similarity between bacterial communities around fresh seeds and under conditions where the components are sterilized. These results clearly indicate that the SRB around Zostera marina seeds create anaerobic conditions that promote germination through their sulfate-reducing function. Generally, H2S produced by sulfate-reducing bacteria is toxic to a variety of organisms, including plants. However, it is now clear that sulfate-reducing bacteria from eelgrass seeds play an essential role in establishing the germination environment of Zostera marina.
Moreover, recent studies have reported that the presence of bivalves in seagrass beds enhances the germination rate and establishment rate of Zostera marina seedlings [26]. This research investigated how the increased biomass of bivalves leads to the decomposition of total organic matter in sediments, resulting in increased total nitrogen and exchangeable ammonia, which in turn improves the seedling height, chlorophyll b content, and superoxide dismutase activity of Zostera marina. These findings reveal that the construction of anaerobic environments through organic matter decomposition by sulfate-reducing bacteria and benthic organisms contributes to the improved germination rate of Zostera marina. This indicates the importance of maintaining healthy bottom material circulation for eelgrass meadow conservation and the need to recreate the lost mechanisms for sustaining biodiversity.
Data availability
The 16S metagenomic sequences for each condition were deposited in DDBJ under BioProject number PRJDB17004 (https://ddbj.nig.ac.jp/resource/bioproject/PRJDB17004), BioSample number SAMD00657117-SAMD00657121 (https://ddbj.nig.ac.jp/resource/biosample/SAMD00657117), and accession number DRA 526471-DRR526475 (https://ddbj.nig.ac.jp/resource/sra-run/ DRA017784).
Conclusion
In conclusion, as a preliminary study on eelgrass conservation, we demonstrated that the utilization of eelgrass meadow sediments can significantly increase germination rates by approximately 6%. This indicates that efficient eelgrass meadow conservation can be achieved by minimizing inorganic sediment inputs and utilizing SRB-rich sediments. These findings suggest a promising alternative for eelgrass meadow preservation and emphasize the importance of using native sediments, including indigenous bacteria, such as SRB, to enhance germination rates and contribute to the long-term preservation of marine environments. However, the condition of bottom sediments is unique to each region, it is assumed that the methods to be used for conservation will be fundamentally different, especially between a bay that is nutrient-poor and a mineral-rich sea adjacent to a mountain.
This work was supported by JSPS KAKENHI (JP18K05695 and JP21K05640), a grant from the Takahashi Industrial and Economic Research Foundation, and the “Innovation Inspired by Nature” Research Support Program from Sekisui Chemical Co., Ltd.
- Moore KA, Short FT, Larkum AWD, Orth RJ, Duarte CM. Eds.; Springer: Dordrecht, The Netherlands, Zostera: Biology, Ecology, and Management. Seagrasses: Biology, Ecology and Conservation. Springer 2006; 354: 361–386.
- Nishijima W, Nakano Y, Hizon-Fradejas AB, Nakai S. Evaluation of substrates for constructing beds for the marine macrophyte Zostera marina L. Ecol. Eng. 2015; 83: 43–48.
- Olsen JL, Rouzé P, Verhelst B, Lin YC, Bayer T, Collen J, Dattolo E, De Paoli E, Dittami S, Maumus F, Michel G, Kersting A, Lauritano C, Lohaus R, Töpel M, Tonon T, Vanneste K, Amirebrahimi M, Brakel J, Boström C, Chovatia M, Grimwood J, Jenkins JW, Jueterbock A, Mraz A, Stam WT, Tice H, Bornberg-Bauer E, Green PJ, Pearson GA, Procaccini G, Duarte CM, Schmutz J, Reusch TB, Van de Peer Y. The genome of the seagrass Zostera marina reveals angiosperm adaptation to the sea. Nature. 2016 Feb 18; 530(7590):331-5. doi: 10.1038/nature16548. Epub 2016 Jan 27. PMID: 26814964.
- Fonseca MS, Kenworthy WJ, Courtney FX, Hall MO. Seagrass Planting in the Southeastern United States: Methods for Accelerating Habitat Development. Restor. Ecol. 1994; 2:198-212.
- Zhou Y, Liu P, Liu B, Liu X, Zhang X, Wang F, Yang H. Restoring eelgrass (Zostera marina L.) habitats using a simple and effective transplanting technique. PLoS One. 2014 Apr 2; 9(4):e92982. doi: 10.1371/journal.pone.0092982. PMID: 24695414; PMCID: PMC3973628.
- van KMM, Thorhaug A, Marbà N, Orth RJ, Duarte CM, et al. Global analysis of seagrass restoration: the importance of large-scale planting. J. Appl. Ecol. 2015; 53: 567-578.
- Wassenberg TJ. Seasonal feeding on Zostera capricorni seeds by Juvenile Penaeus esculentus (Crustacea: Decapoda) in Moreton Bay, Queensland. Aust. J. Mar. Freshw. Res. Research 1990; 41: 301-310.
- Orth RJ, Luckenbach M, Moore KA. Seed Dispersal in a Marine Macrophyte: Implications for Colonization and Restoration. Ecology 1994; 75: 1927-1939.
- Fishman JR, Orth RJ. Effects of predation on Zostera marina L. seed abundance. J. Exp. Mar. Biol. Ecol. 1996; 198: 11-26.
- Orth RJ, Marion SR, Granger S, Traber M. Evaluation of a mechanical seed planter for transplanting Zostera marina (eelgrass) seeds. Aquat. Bot. 2009; 90:204-208.
- Tanner EC, Parham T. Growing Zostera marina (eelgrass) from seeds in Land-Based Culture Systems for use in restoration projects. Restor Ecol. 2010; 18: 527-537.
- Kusube M. Research on innovative marine environmental conservation with no environmental impact using marine bio-cement. Impact 2020; 3: 57-59.
- Morita T, Miyamatsu A, Fujii M, Kokubu H Abe M, et al. Germination in Zostera japonica is determined by cold stratification, tidal elevation and sediment type. Aquat. Bot. 2011; 95: 234-241.
- Nakashima Y, Sonobe T, Hanada M, Kitano G, Sonoyama Y, Iwai K, Kimura T, Kusube M. Microbial Detoxification of Sediments Underpins Persistence of Zostera marina Meadows. Int J Mol Sci. 2024 May 16; 25(10):5442. doi: 10.3390/ijms25105442. PMID: 38791480; PMCID: PMC11122150.
- Morita T, Kakinuma M, Mizuno G, Okumura I, Kokubu H, et al. Morphological characteristics of annual Zostera marina shoots at various germination temperatures. Aquat. Bot. 2010; 92: 49-54.
- Xu S, Zhou Y, Wang P, Wang F, Zhang X, Gu R. Salinity and temperature significantly influence seed germination, seedling establishment, and seedling growth of eelgrass Zostera marina L. PeerJ. 2016 Nov 15; 4:e2697. doi: 10.7717/peerj.2697. PMID: 27896031; PMCID: PMC5119234.
- Chen J, Hanke A, Tegetmeyer HE, Kattelmann I, Sharma R, Hamann E, Hargesheimer T, Kraft B, Lenk S, Geelhoed JS, Hettich RL, Strous M. Impacts of chemical gradients on microbial community structure. ISME J. 2017 Apr; 11(4):920-931. doi: 10.1038/ismej.2016.175. Epub 2017 Jan 17. PMID: 28094795; PMCID: PMC5363838.
- Wang S, Yan Z, Wang P, Zheng X, Fan J. Comparative metagenomics reveals the microbial diversity and metabolic potentials in the sediments and surrounding seawaters of Qinhuangdao mariculture area. PLoS One. 2020 Jun 4; 15(6):e0234128. doi: 10.1371/journal.pone.0234128. PMID: 32497143; PMCID: PMC7272022.
- Rognes T, Flouri T, Nichols B, Quince C, Mahé F. VSEARCH: a versatile open source tool for metagenomics. PeerJ. 2016 Oct 18; 4:e2584. doi: 10.7717/peerj.2584. PMID: 27781170; PMCID: PMC5075697.
- Beals WE. Bray-Curtis ordination: An effective strategy for analysis of multivariate ecological data. Adv. Ecol. Res. 1984; 14: 1-55.
- Jørgensen BB, Findlay AJ, Pellerin A. The Biogeochemical Sulfur Cycle of Marine Sediments. Front Microbiol. 2019 Apr 24; 10:849. doi: 10.3389/fmicb.2019.00849. PMID: 31105660; PMCID: PMC6492693.
- Hootsmans MJM, Vermaat JE, Van Vierssen W. Seed bank development, germination and early seedling survival of two seagrass species from the Netherlands: Zostera marina L. and Zostera noltii Hornem. Aquat.Bot. 1987: 28: 275-285.
- Kushkevych I, Hýžová B, Vítězová M, Rittmann SKR. Microscopic Methods for Identification of Sulfate-Reducing Bacteria from Various Habitats. Int J Mol Sci. 2021 Apr 13; 22(8):4007. doi: 10.3390/ijms22084007. PMID: 33924516; PMCID: PMC8069399.
- Fahimipour AK, Kardish MR, Lang JM, Green JL, Eisen JA, Stachowicz JJ. Global-Scale Structure of the Eelgrass Microbiome. Appl Environ Microbiol. 2017 May 31; 83(12):e03391-16. doi: 10.1128/AEM.03391-16. PMID: 28411219; PMCID: PMC5452814.
- Sun Y, Song Z, Zhang H, Liu P, Hu X. Seagrass vegetation affect the vertical organization of microbial communities in sediment. Mar Environ Res. 2020 Dec; 162:105174. doi: 10.1016/j.marenvres.2020.105174. Epub 2020 Oct 7. PMID: 33099080.
- Bowen R, Huizhen C, Xiangtao W, Weihao B, Weidong G, et al. Promoting effect of Manila clam ruditapes philippinarum (Adams & Reeve, 1850) on the establishment of eelgrass Zostera marina L. seedlings. Reg. Stud. Mar. Sci. 2024; 73: 103491.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
