Annals of Marine Science
Fluorescent analysis of fish larvae of Engraulis encrasicholus ponticus L.
Victoria V Roshchina1*, Tatyana N Petrova2 and Vladimir I Maltsev2
2T.I. Vyazemsky Karadag Scientific Station – Nature Reserve of RAS – Branch of A.O. Kovalevsky Institute of Biology of the Southern Seas of RAS, Nauki str., 24, Kurortnoye Settlement, Feodosia, 298188 Crimea, Russia
Cite this as
Roshchina VV, Petrova TN, Maltsev VI (2024) Fluorescent analysis of fish larvae of Engraulis encrasicholus ponticus L. Ann Mar Sci 8(1): 008-010. DOI: https://dx.doi.org/10.17352/ams.000043Copyright License
© 2024 Roshchina VV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.The fluorescence after histochemical reactions for biogenic amines of the fish larva of European anchovy (Engraulis encrasicholus ponticus L.) from the Black Sea has been analysed as possible methodical testing of the stage of development and vitality. The purpose of the study is to use their testing in ecological monitoring resources of the sea reservation and fishery. The histochemical staining for dopamine, histamine, and serotonin permitted us to estimate the state of the single larva based on their fluorescence. Observers may see the internal structure of the larva cell tissues under the excitation of ultraviolet, violet, and green light. This approach should be recommended for practice in the industrial fishery. as possible testing of this stage of development and state in the ichthyoplankton system.
Introduction
New possibilities were estimated when using luminescence microscopy with modifications such as a microspectrofluorimeter that permitted to record spectra from the various parts of an egg cell from the examples of the Black Sea red mullet roe Mullus barbatus ponticus Essipov., greater weever Trachinus draco L., Black sea horse-mackerel Trachurus meditterraneus ponticus Aleev. Or European anchovy Engraulis encrasicholus ponticus L. [1,2]. The publications were linked mainly with earlier stages of fish egg development [1,2]. Unlike the eggs of the first three species, the popular fish European anchovy had no strong eggs or larvae autofluorescence, except fluorescence after histochemical reactions for biogenic amines [1]. However, there may be a chance that more intensive was the fluorescence in the fish larva test analysis. Fluorescent methods were not used in similar practice yet, and we had the purpose of performing this for fish larva European anchovy Engraulis encrasicholus ponticus L.
Materials and methods
The objects of research were larvae of European anchovy Engraulis encrasicholus ponticus L that belongs to the family Engraulidae and is ordinary for the fishery in the Black Sea basing near Karadag Biological Station of Feodosia, Russia [3-5]. Samples of ichthyoplankton were fixed with 4% or 10% formalin, the larvae were determined and chosen. Whole larvae were saved some days before the laboratory analysis. Undamaged larvae (without any physical dissection of the structure) were used for undergoing the microscopic observation.
Measurement of fluorescence
Autofluorescence of samples in 10% formalin was observed and photographed directly on slides [1,2] using a Leica DM 6000 B luminescent microscope (Germany) and the MSF-15 microspectrophotometer/fluorimeter (LOMO, Russia) [1] with the Levenhuk M300 Base camera (USA). The excitation of fluorescence before and after histochemical staining for biogenic amines was at ultraviolet 360 nm - 380 nm, violet 430 nm - 450 nm, and green 480 nm - 500 nm.
Histochemical reactions for determination of biogenic amines
Fluorescent histochemical determination of biogenic amines (dopamine, histamine, and serotonin) within cells, was carried out according to the methods primarily described for animal cells [6] and applied for fish egg cells as well [1]. Larvae were put on object glasses (slides) and moistened with drops of 1% aqueous solutions of glyoxylic acid for dopamine, o-phthalic aldehyde for histamine, or formaldehyde for serotonin. After 10 - 20 minutes of staining with the reagent, samples were dried at 50 °C – 80 °C during 5 - 10 minutes. Fluorescence reactions of forming products were studied under the luminescence microscope Leica DM 6000 B or by camera Levenhook (USA) at the excitation by light 360-380 nm. The fluorescence spectra were recorded by microspectrofluorimeter MSF-15 (LOMO, Sankt-Petersburg). Histochemical reactions repeated (up to 3-5 times). Results of the fluorescence intensity at 460 nm in the larva head, eye, and body (relative units of microspectrofluorimeter) were expressed statistically with a standard error of mean +SEM (n = 4 - 5 subject glasses with 3 cells).
Results and Discussions
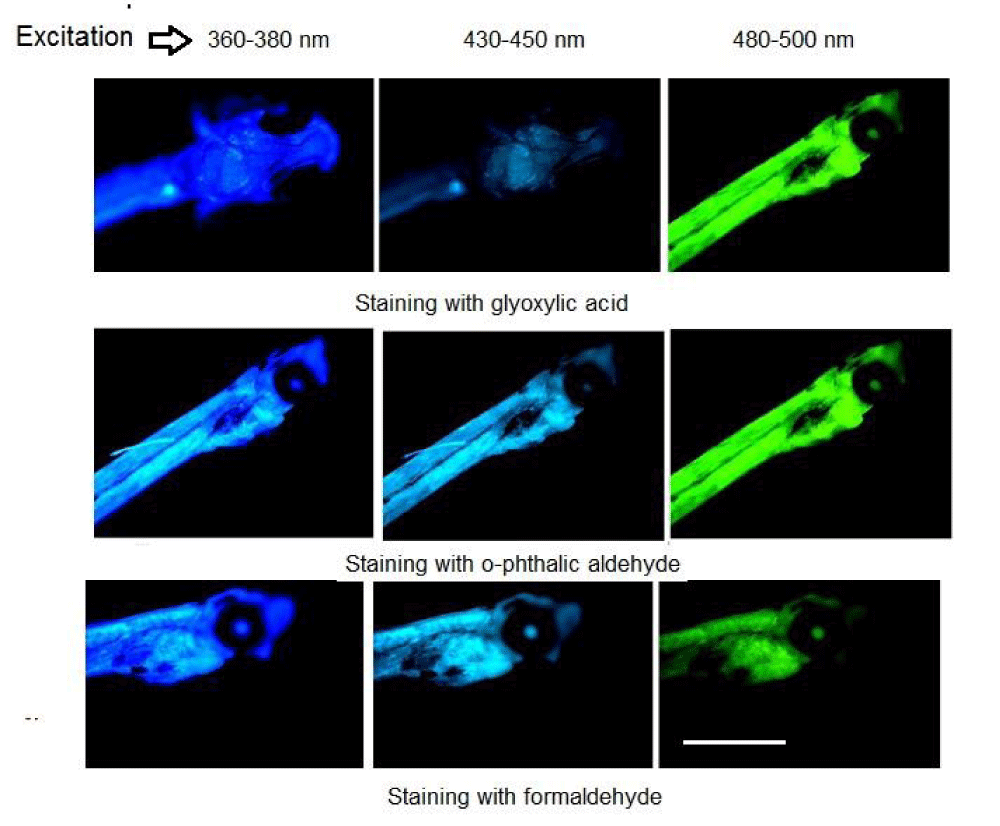
The autofluorescence of larvae studied looked weakly at any excitation of the luminescence microscope and we tried to use a histochemical approach for biogenic amines. In this case, the staining with glyoxylic acid, o-phthalic aldehyde, and formaldehyde for the determination of dopamine, histamine, and serotonin, relatively [1,6], led to the enhancement of the emission at various light excitation (Figure 1). Usually the staining with reagents for biogenic amines are analysed at ultraviolet irradiation and seen emission well at range 450 nm - 470 nm [1,6]. We observed the blue fluorescence image, but under ultraviolet excitation 360 nm - 380 nm of luminescence microscope details were not well-seen (Figure 1). To improve the observation, the emission from the larva was seen under the excitation at longer wavelengths (Figure 1). In this case, bright emission was received, mainly in fish larva head and the body as a whole. This approach permitted the observation of a well-lighted head and even eye, which was not seen under UV-excitation. They became visible as light eye spots surrounded with dark unfluoresced structures only after the excitation at 480 nm - 500 nm in the staining with glyoxylic acid (Figure 1). When carrying out staining with o-phthalic aldehyde, reagent for histamine, or with formaldehyde, reagent for serotonin, we observed brighter emission of the body and eyes, even at UV-excitation. Although green effects for glyoxylic acid and o-phthalic aldehyde seem to be similar, however, fluorophores formed at the histochemical reactions are different products [6]. We observed the phenomenon of different emissions in violet and especially in green as well, at other excitations of the same probes excited with longer wavelengths. In the picture observed in the case of the staining for serotonin, the larva swelled in the head part. At the fluorescence observation, one can estimate, how the larva is developed.
From the emission spectra at 450 nm - 470 nm (excitation 360 nm - 380 nm) recorded as described earlier [1], we composed Table 1, based on the fluorescence. It could estimate the content of biogenic amines in the single larva, including the body as a whole, head, and eye based on the fluorescence intensity recorded by microspectrofluorimeter after the staining with specific reagents for biogenic amines minus the autofluorescence. We could use microspectrofluorimeter optical probes with different sizes of 2 μm - 20 μm for various parts of the object. Table 1 shows that the larva head has much more biogenic amines, than the eye. The large amount of dopamine and histamine in larvae appears to be a sign of cellular damage. The scientists may work with a special standard for a similar determination to use the calibration curve for any microspectrofluorimeter.
In our short report, we mark the following possibilities for laboratory and fishery. The use of reagents for biogenic amines for fluorescent reactions permits us to see both the presence of the neurotransmitters as well as to receive better images of whole fish larva without a dissection of the object. It is of great value because the larvae of our object are colorless. Earlier we saw the method for the first steps of the larva appearance directly in a fish egg [1]. Today it is also applied for liberated larva out of the cell. Unlike the application of the markers of nucleic acids [7] that widely described in literature from 1980 to nowadays or the observation of the natural fluorescence with microcapsules on fish larvae [8], this approach with microspectrofluorometry has a perspective for the observation of ichthyoplankton without artificial procedures with fluorescent molecular probes or fluorescent proteins either in a sea reservation or in the fishery industry.
Conclusion
The fluorescent method based on the staining for biogenic amine reactions may allow us to estimate the stages of the fish larvae’s development and vitality in the future. Observers may see the internal structure of the larva cell tissues under the excitation of ultraviolet, violet, and green light. This approach with microspectrofluorometry has a perspective for the observation of ichthyoplankton either in a sea reservation or in the fishery industry without artificial procedures with fluorescent molecular probes or fluorescent proteins.
We are grateful to Optical Microscopy and Spectrophotometry core facilities, ICB RAS, Federal Research Center “Pushchino Scientific Center for Biological Research of the Russian Academy of Sciences for the use of Leica microscopes.
Absolute author contribution
Common idea, all experiments and the paper writing (Roshchina V.V.), receiving of larvae in fish eggs plankton and identification as well as discussion (Petrova T.N. and Maltsev V.I.).
- Roshchina VV, Yashin VA, Petrova TN, Maltsev VI. Fluorescent Methods for Test-Analysis of Plankton Fish Eggs. EC Microbiology. 2023; 19(1):34-38.
- Roshchina VV, Yashin VA, Petrova TN, Maltsev VI. Fluorescence of plankton fish eggs of Black Sea Mullus barabatus ponticus for test-analysis of the cell fertilization and development. Ann Mar Sci. 2024; 8(1):002-007.
- Dekhnik TV. Ichthyoplankton of Black Sea. Kiev: Naukova Dumka; 1973; 237.
- Maltsev VI. Coastal ichthyocomplex of specially protected waters of the south-eastern Crimea. In: Marine Technologies: Problems and Solutions – 2021: a collection of articles by participants of the National Scientific and Practical Forum. Edited by Masyutkina EP. Conf. Kerch, April 19-30, 2021. Kerch: KSMTU; 2021; 277–280.
- Maltsev VI, Vasilets VE, Shaganov VV, Petrova N. Revision of the species composition of fish of the coastal ichthyocomplex of the water area of the Karadag Nature Reserve. Bull Kerch State Mar Tech Univ. 2021; (2):50-65.
- Markova LN. Histochemical study of biogenic monoamines in early ("Prenervous") and late embryos of sea urchins. Int J Dev Neurosci. 1985; 3(5):493-495.
- Clemmensen C. A RNA and DNA fluorescence technique to evaluate the nutritional condition of marine fish larvae. Meeresforsch Reports Mar Res. 1988; 32:134-143.
- Lee IS. Determining live prey preferences of larval ornamental marine fish utilizing fluorescent microspheres. Aquaculture. 2018; 490(1):125-135.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
