Annals of Marine Science
Identification and characterization of marine pathogenic vibrios in cultured golden pompano (Trachinotus ovatus) in Guangxi, China
Qing Yu1#, Mingzhu Liu1#, Fei Li1, Yibing Wang4, Zijun Lu4, Siting Wu1, Xianling Qin2, Xianyun Chen2, Deqiang Shi1, Lantian Lu1, Ke Ke1, Qiwei Qin2* and Pengfei Li1,3*
2College of Marine Sciences, South China Agricultural University, Guangzhou 510642, China
3Guangxi Key Laboratory of Marine Environmental Science, Guangxi Academy of Sciences, Nanning, P.R. China
4College of Marine Sciences, Guangxi University for Nationalities, Nanning, P.R. China
##These authors contributed equally to this work.
Cite this as
Yu Q, Liu M, Li F, Wang Y, Li P, et al. (2018) Identification and characterization of marine pathogenic vibrios in cultured golden pompano (Trachinotus ovatus) in Guangxi, China. Ann Mar Sci 2(1): 016-019. DOI: 10.17352/ams.000010Vibriosis has caused great economic losses to marine aquaculture with high mortality worldwide. Guangxi province is an important cultured center for tropical marine fish species in South China. However, no research focused on epidemiological characterization of vibriosis in golden pompano aquaculture in Guangxi province have been carried out. We isolated and identified the strains of V. harveyi caused the serious disease that occurred in cultured golden pompano in Guangxi province, by biochemical and physiological tests, and PCR. A cell line derived from the brain of golden pompano Trachinotus ovatus (TOGB) had been established and characterized in the previous study, was applied to analyze the cytotoxicities of extracellular products (ECPs) from GT-V. harveyi isolates. We also proved that GT-V. harveyi isolates produced cytotoxic effects in vivo. This study identified the vibriosis outbreaks in golden pompano aquaculture in Guangxi province this year.
Introduction
As one of the most devastating and prevalent diseases in marine aquaculture, vibriosis has caused great economic losses with with high mortality worldwide [1]. Vibrio species could cause serious diseases in various cultured tropical fish, such as golden pompano (Trachinotus ovatus), tilapia (Oreochromis sp), eel (Anguilla anguilla) and trout (Oncorhynchus mykiss) [2]. The distribution of Vibrio has recently been extended due to global warming, which brings about higher prevalence of pathogenic vibrios and more potential harms in aquatic environments [3]. Some Vibrio species are also opportunistic human pathogens, which could infect humans through skin contact or injuries and cause serious diseases [4]. The current control strategies against vibriosis are mainly focusing on chemotherapy and vaccination [5]. However, the abuse of antibiotics has caused the occurrence of multidrug resistant strains, which also poses high risks to human health [6]. Golden pompano Trachinotus ovatus, Carangidae, Perciformes, is a commercially important cultured marine fish and widespread across in China, Japan and some other Asian countries [7]. The golden pompano aquaculture industry grown rapidly in recent decades, but is seriously threatened by pathogens, such as bacteria, virus and parasite, which have caused great economic losses [8]. Especially the bacterial diseases caused by pathogenic vibrios, they come on quickly with high incidence rates and fatality rates. Guangxi province is an important cultured center for tropical marine fish species in South China. However, no research focused on epidemiological characterization of vibriosis in golden pompano aquaculture in Guangxi province have been carried out. In this study, the epidemiological investigation of vibriosis was undertaken to isolate and determine pathogenic Vibrio species in golden pompano aquaculture in Guangxi province.
Materials and Methods
Sample collection and Vibrio strains isolation
The outbreaks of suspected vibriosis emerged in golden pompano cultured in southern coastal area of Guangxi province in May in 2018. The fish were kept in ice-storage boxes and sent to the laboratory in Nanning City for pathogenic vibrio detection and isolation immediately. For isolation of Vibrio strains, samples were taken from external lesions, liver, spleen and kidney of diseased fish. After being cut into pieces in phosphate-buffered saline (PBS), 100μl serial dilutions were plated on the thiosulfate citrate bile salt sucrose agar (TCBS) and incubated at 37°C for 24 h. Totally 5 suspected vibrio strains were isolated and pure cultured by streaking on TCBS agar plates, and further characterized to determine if they were vibrios by biochemical and physiological tests.
Biochemical and physiological analysis
The biochemical and physiological tests of isolated vibrio strains were carried out by bacteria biochemical identification tubes (HKM, Guangzhou, Guangdong, China), including colony morphology and pigmentation on TCBS, gram-staining, cell morphology, gas from glucose, Voges-Proskauer, sucrose, mannose, arabinose, inositol, arginine dihydrolase and lysine decarboxylases. All tests were performed at 37°C. The growth characteristics of salinity (0, 1, 3, 6, 8 and 10% NaCl, w/v) were determined on TSB. The biochemical identification tubes were observed up to 10 days before being determined as negative.
16S gene analysis and phylogenetic tree
A pair of 16S primers (F-primer, 5’-GAGTTTGATCCTGGCTCAG-3’ and R-primer, 5’-CGGTTACCTTGTTACGACTT-3’) was used to amplify the 16S gene fragments of isolated Vibrio strains according to the instruction of PrimeSTAR HS DNA polymerase (TaKaRa). Thermal cycling of PCR amplification was performed using 30 cycles of denaturation at 95°C for 1 min, annealment at 55°C for 45 s, and extension at 72°C for 1 min, followed by a final extension at 72°C for 10 min. The PCR fragments were detected by by agarose gel electrophoresis, and the fragments of 16S gene were sequenced by an ABI 3730 DNA analyser. BLASTN and BLASTP (NCBI database) were applied to assemble and analyse the obtained 16S rRNA sequences of isolated Vibrio strains. The phylogenetic tree was constructed by the neighbour-joining method by Molecular Evolutionary Genetics Analysis as described previously with some modifications [9].
Cytotoxicity analysis of GT-V. harveyi isolates
The cytotoxicities of extracellular products (ECPs) from GT-V. harveyi were analyzed in TOGB cells (Li et al., 2016). GT-V. harveyi isolates were inoculated in 2 ml saline tryptone soya broth at 28°C for 48 h, respectively. After being centrifuged by 12000 g at 4°C for 20 min, the supernatants were filtered through 0.22 µm filter (EMD Millipore, Billerica, MA, USA) to collect extracellular product preparations. Total protein concentrations of the supernatants were measured by BCA protein assay (Pierce) standardized to bovine serum albumin (BSA), according to the manufacturer’s protocol. 100 μL cells per well (at a concentration of 3×105/mL) were seeded in 96-well plates (Shengyou Biotechnology, Hangzhou, China) at 28°C for 24 h, and then incubated with 10 μL ECPs (100, 500, 1000 μg/mL) at 28°C for 24 h. The cells were then examined by light microscopy to identify the signs of cytotoxicity. TOGB cells incubated with 10 μL PBS served as the control group. The cell viability in each well was also assessed based on assays described previously, with some modifications. TOGB cells were incubated with 10 μL of ECPs (100, 500, 1000 μg/mL) of GT-V. harveyi isolates at 28°C for 24 h. Cell viability was measured by modified MTT assay (Yu et al. 2009). 10 μL WST-8 (Dojindo, Kumamoto, Japan) was added to cultured cells in each well and incubated at 28°C for 3 h. The absorbance of each well at 450 nm was measured using a microplate reader (Thermo, Waltham, MA, USA).
Virulent capacities of GT-V. harveyi isolates in vivo
The virulent capacities of the isolates were further analyzed. Prior to challenge, fish were randomly sampled and subjected to microbiological analysis, which indicated that they were free of pathogens. Golden pompano (15g each), were starved for 24 h before receiving a 100 μl intraperitoneal injection containing 108 CFU/ml GT-V. harveyi isolates. The fish in the control groups were injected with the same amount of PBS. Twenty fish were used in each treatment group. Each group was transferred to a separate aquarium supplied with running seawater and ample aeration, and maintained in 26‰ salinity seawater in tanks with a closed, circulating nonchlorinated-water system, and fed a daily diet of commercially available food. Mortality was recorded daily until day 10 post-injection. Dead fish were collected for microbiological analysis for re-isolation of the inoculated strain. The Ethics Committee for Animal Experiments of Guangxi Academy of Sciences approved the protocols of this study.
We also analyzed the drug resistance of these GT-V. harveyi isolates. Resistance against 18 antimicrobial agents was tested by Kirby-Bauer antibiotic susceptibility testing [10].
Results and Discussion
Totally 5 suspected vibrio strains were isolated and pure cultured by streaking on TCBS agar plates. The biochemical and physiological results showed that, compared to ATCC Type strains of V. harveyi originally isolated from sea water (ATCC 25919) [11,12], these bacterial isolates were Gram-negative and produced yellow (1, 3, 5) or green (2, 4) colonies on TCBS agar (Figure S1). These bacterial isolates had typical characteristics of marine vibrios, such as motile, catalase and oxidase positive, presented glucose fermentative metabolism without gas production and were sensitive to the vibriostatic agent O/129 (150 μg) [9]. They could grow in the presence of 1-8% NaCl, while the optimum salinity is 3-6%. Compared to ATCC Type strains of V. harveyi (ATCC 25919) and the isolate 1, the other isolates (1, 2, 3, 4) failed to utilize sucrose and were lysine decarboxylase-negative. Other biochemical properties are shown in Supplementary table S1. The present results suggested that there may be more than one strain of V. harveyi caused the serious disease that occurred in cultured golden pompano in Guangxi province in May this year.
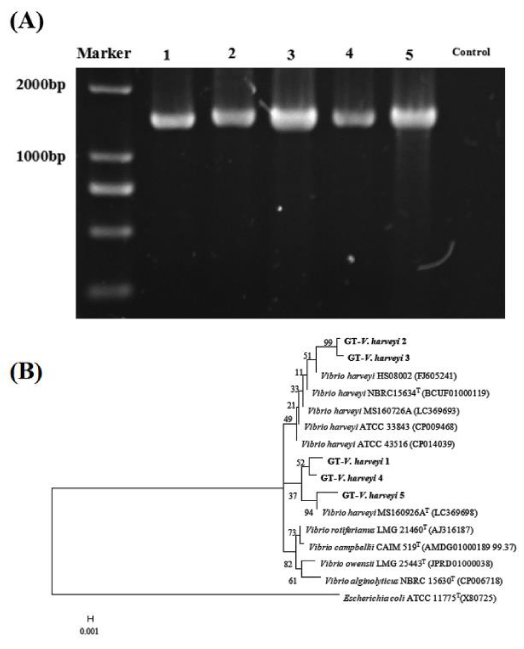
The expected 16S rRNA PCR fragments of isolated Vibrio strains (1466 bp) were obtained (Figure 1A). The phylogenetic tree results showed that vibrios isolates from golden pompano in Guangxi province were Vibrio harveyi, whose similarity was up to 99% (Figure 1B). Therefore Vibrio harveyi isolated from diseased golden pompano Trachinotus ovatus, which were reared in Guangxi province, and tentatively designated as Guangxi Trachinotus ovatus Vibrio harveyi (GT-V. harveyi 1-5).
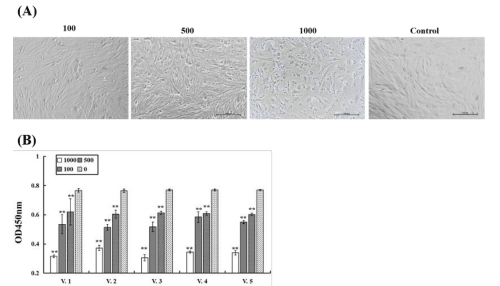
The ECPs cytotoxicity results showed that, compared to control groups (Control), morphological changes were detected in TOGB cells incubated with ECPs for 48 h, including becoming round, shrinking, and separating from the culture plate (Figure 2A). The cell viability results showed that, compared to controsl groups, cell viability decreased with ECPs, indicating the cytotoxicity of GT-V. harveyi isolates (Figure 2B).
The virulent capacities of the isolates were also analyzed. Among the golden pompano treated with GT-V. harveyi isolates, the cumulative mortality reached 100% from day 5 to day 9 postinfection, respectively, while no fish were dead in the control groups (Figure 3A). The dead fish had similar symptoms as previously naturally diseased fish, such as skin and muscle ulceration, internal organs swelling and congestion. The 16S gene PCR results proved that, the bacterial strains isolated from the diseased fish were V. harveyi. After being fixed in 10% neutral buffered formalin and embedded in paraffin, tissue specimens were stained with hematoxylin and eosin (H&E). Compared to the control group, the histological results revealed obvious pathological changes in the liver and kidney of GT-V. harveyi isolates-injected fish, suggesting that the GT-V. harveyi isolates produced cytotoxic effects in vivo (Figure 3B).
The drug resistances of GT-V. harveyi isolates were showed in table 1, GT-V. harveyi 2, GT-V. harveyi 4 and GT-V. harveyi 5 showed multiple resistance to the tested drugs, which were resistant to 10, 6 and 4 antibiotics, respectively. Penicillin, erythromycin, acheomycin, rifampicin, ciprofloxacin and roxithromycin showed low efficacy against V. harveyi isolates. In order to avoid the acquisition of resistance by aquatic pathogens, we have suggested the aquaculture farmers to improve the culture environment, prevent vibrio fish diseases by injecting vaccines, and choose other highly sensitive drugs if necessary.
Taken together, this study identified the vibriosis outbreaks in golden pompano aquaculture in Guangxi province this year. We would apply the isolated Vibrio harveyi for developing rapid convenient diagnosis assay and effective regionally customized vaccines for V. harveyi infection in Guangxi mariculture.
This work was supported by grants from the Key Research and Development Programs of Guangxi (2018AB52003), the Natural Science Foundation of Guangxi (2017GXNSFBA198176), Basic research fund of Guangxi Academy of Sciences (2017YJJ23002), Opening Project of Guangxi Laboratory on the Study of Coral Reefs in the South China Sea (GXLSCRSCS2018003). We would like to thank International Science Editing to language edit my paper.
- Tan D, L Gram and M Middelboe (2014) Vibriophages and their interactions with the fish pathogen Vibrio anguillarum. Appl Environ Microbiol 80: 3128-40. Link: https://tinyurl.com/yahupppn
- Amaro C, E Sanjuán, B Fouz, D Pajuelo, CT Lee, et al. (2015) The Fish Pathogen Vibrio vulnificus Biotype 2: Epidemiology, Phylogeny, and Virulence Factors Involved in Warm-Water Vibriosis Microbiol Spectr 3. Link: https://tinyurl.com/yabucfjq
- Böer S, EA Heinemeyer, K Luden, R Erler, G Gerdts, et al. (2013) Temporal and spatial distribution patterns of potentially pathogenic Vibrio spp. at recreational beaches of the German North Sea. Microb Ecol 65: 1052-1067. Link: https://tinyurl.com/ybtljbam
- Horseman, MA and S Surani (2011) A comprehensive review of Vibrio vulnificus: an important cause of severe sepsis and skin and soft-tissue infection. Int. J. InfectDis 15: e157-e166. Link: https://tinyurl.com/ycsnvkav
- Li P, Q Yu, X Qin, F Li, X Chen, et al. (2018) Current situation and research prospects of disease control technology system of mariculture in Beibu Gulf,Guangxi. Guangxi Sciences 25: 15-25. Link: https://tinyurl.com/yc86q7t3
- Akinbowale OL, H Peng and MD, Barton (2006) Antimicrobial resistance in bacteria isolated from aquaculture sources in Australia J Appl Microbiol 100: 1103-1113. Link: https://tinyurl.com/y75uu36a
- Zhou L, P Li, S Ni, Y Yu, M Yang, et al. (2017) Establishment and characterization of a mid-kidney cell line derived from golden pompano Trachinotus ovatus, a new cell model for virus pathogenesis and toxicology studies. In Vitro Cell Dev Biol Anim 53: 320-327. Link:
https://tinyurl.com/ycmzrcp7 - Li P, L Zhou, M Yang, S Ni, Y Yu (2017) Establishment and characterization of a cell line from the head kidney of Golden pompano (Trachinotus ovatus) and its application in toxicology and virus susceptibility. J Fish Biol 90: 1944-1959. Link: https://tinyurl.com/ycn49fsw
- Lasa A, R Avendaño-Herrera, JM Estrada and JL Romalde (2015) Isolation and identification of Vibrio toranzoniae associated with diseased red conger eel (Genypterus chilensis) farmed in Chile. Vet Microbiol 179: 327-31. Link: https://tinyurl.com/y99n97p9
- Gerbig DG, J Engohang-Ndong and H Aubihl (2013) A new twist to the Kirby-Bauer antibiotic susceptibility test activity-Increasing antibiotic sensitivity of pseudomonas fluorescens through thermal stress. J Microbiol Biol Educ 14: 269-70. Link: https://tinyurl.com/ycr96wra
- Liu PC, KK Lee, KC Yii, GH Kou and SN Chen (1996) Isolation of Vibrio harveyi from diseased kuruma prawns Penaeus japonicus. Curr Microbiol 33: 129-32. Link: https://tinyurl.com/y7vflwvz
- Joosten P, E Tiemersma, A Threels, C Caumartin Dhieux and J Rombout (1997) Oral vaccination of fish against Vibrio anguillarum using alginate microparticles. Fish Shellfish Immunol 7: 471-485. Link: https://tinyurl.com/ydftu89jnces

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
